Contents
Epigenetics
Introduction
Not all characteristics of living organisms can be explained by their genes: factors other than the primary DNA sequence are important. Epigenetics, information that is heritable during cell division other than the DNA sequence itself, is the subject of considerable current interest.
In multicellular organisms, all cells apart from the gametes (sex cells) possess the same genetic content, but may perform very different functions. Cells in different parts of the body differ in the number of active genes — genes that are switched on (and expressed). Genes that are not needed in a particular cell are switched off (silenced) during development. Epigenetics, not genetics, discriminates between different types of cells in an organism. Specific epigenetic marks dictate whether particular genes are expressed.
The modification of chromatin (the tightly packed complex of proteins and genomic DNA) is responsible for epigenetic regulation of gene expression. Like genetic changes, epigenetic changes are preserved when a cell divides. A cell's epigenome is the overall epigenetic state of a cell.
Molecular basis of epigenetics: DNA modification and histone modification
The best-studied epigenetic modification is the methylation of DNA at cytosine bases. Cytosine methylation indicates gene silencing: methylated genes are not transcribed.
Histone modifications also play an important role in epigenetic regulation. Histones are highly basic proteins that act as spools around which DNA winds in chromatin. This compacting allows the DNA to fit into a much smaller space than it would otherwise. Histones possess long N-terminal tails composed mainly of basic amino-acids residues (histidine, lysine and arginine). Post-translational epigenetic modification of the basic side-chains in histone tails takes the form of methylation, acetylation, phosphorylation and ubiquitinylation.
DNA modifications typically correspond to long-term epigenetic memory: once methylated, genomic DNA remains methylated through generations. Histone modifications, on the other hand, typically provide short-term epigenetic memory and can be reversed after a few cell division cycles.
DNA modification
DNA methylation
The methylation of cytosine to 5-methylcytosine (mC, Figure 1) is so important that 5-methylcytosine has been described as the 'fifth base' in the genome.
Methylation occurs at CpG sites (or CpG dinucleotides, i.e. C followed by G), but CpG islands (short stetches DNA with a high concentration of CpG dinucleotides) are generally not methylated in normal tissue. Typically, most of the CpG sites in the genome (outside CpG islands) are methylated. DNA methylation prevents transcription via several mechanisms, including inhibition of transcription factor binding.
Methylation does not affect the base-pairing of cytosine: 5-methylcytosine still forms Watson-Crick base pairs with guanine.
Inheritance of DNA methylation patterns
Replication of methylated DNA results in hemimethylated DNA in which the parent strand is methylated while the daughter strand is unmethylated. Methylation of this hemimethylated DNA is necessary to complete the replication of methylated DNA.
DNA methylation is carried out by DNA methyltransferase enzymes. At least three independent DNA methyltransferases (DNMTs) are involved in DNA methylation. DNA methyltransferases fall into two categories: de novo methyltransferases (DNMT3A and DNMT3B) methylate unmethylated DNA, while maintenance methyltransferases (DNMT1) methylate hemimethylated DNA (Figure 2).
The maintenance methylation of hemimethylated DNA provides a mechanism for inheritance of a methylation pattern through generations, making DNA methylation a stable epigenetic modification.
Mechanism of cytosine methylation
The general mechanism of methylation of cytosine involves electrophilic attack by the cofactor S-adenosyl-l-methionine (AdoMet; SAM), which transfers a methyl group to C(5) of cytosine, and is converted to S-adenosyl-l-homocysteine (AdoHcy) in the process. As the C(5) atom of cytosine is not particularly nucleophilic, some help is needed from the methyltransferase to activate it and increase its nucleophilicity.
The mechanism of cytosine methylation is illustrated in Figure 3. DNA methyltransferases contain a conserved cytosine residue which, on deprotonation to the thiolate anion, acts as a strong nucleophile. The cysteine thiolate attacks the C(6) atom of cytosine in a conjugate addition reaction, and a covalent bond is formed between the cysteine sulfur atom and the cytosine C(6) atom. The negative charge on cytosine is stabilized by interaction with a glutamate residue. Nucleophilic attack then takes place on the methyl group of S-adenosyl-l-methionine, which is converted to S-adenosyl-l-homocysteine (AdoHcy). Finally, β-elimination occurs across the C(5)-C(6) bond, releasing the enzyme.
In the mechanism of methyltransferase-catalyzed methylation of cytosine, a base is required to deprotonate the cysteine to form the (more nucleophilic) thiolate. It is proposed that the base involved in this reaction is a DNA phosphate group, via a bridging water molecule. Therefore, DNA acts as both a substrate and a cofactor (Zangi, 2010).
Role of base flipping in DNA methylation
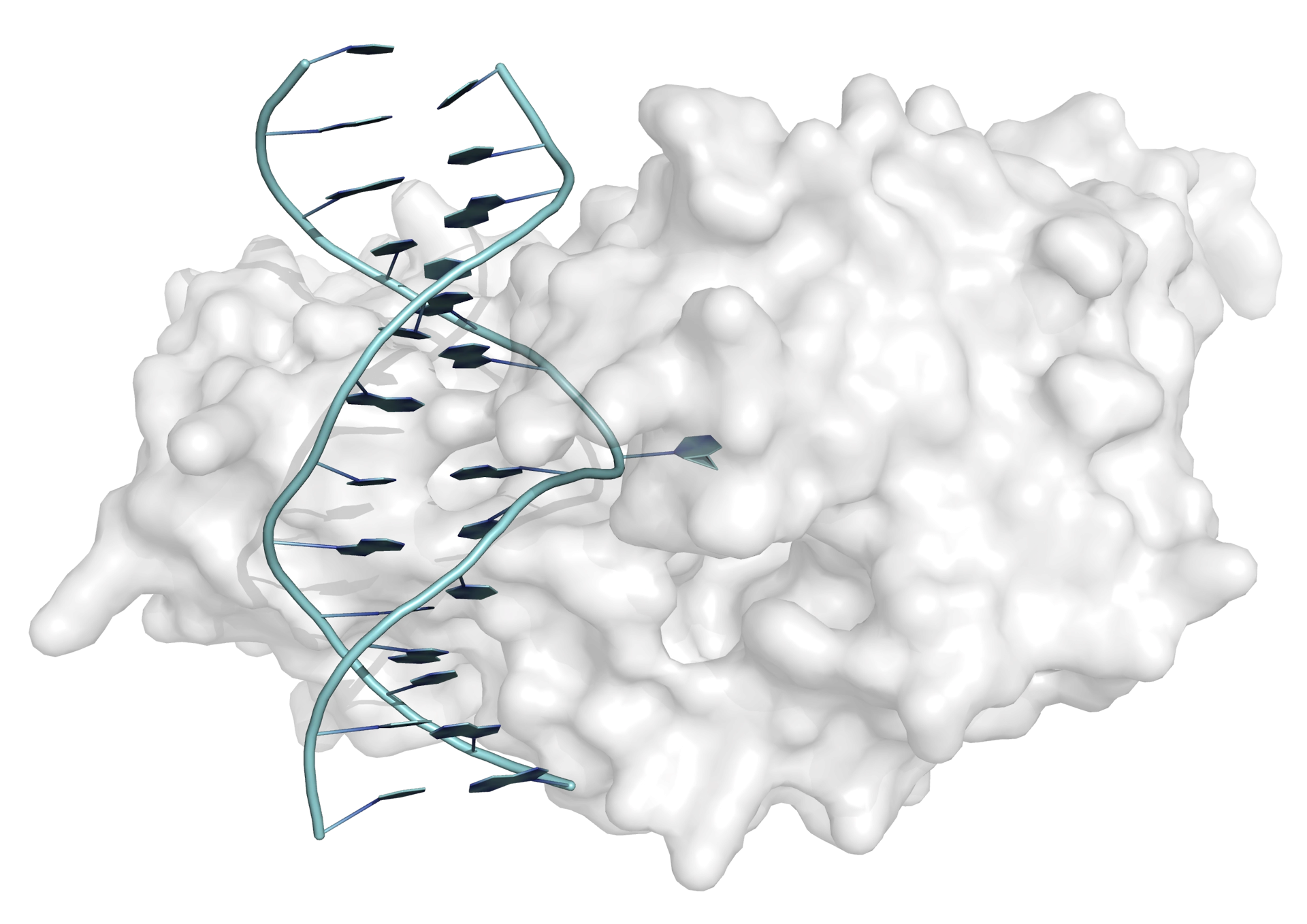
Whenever a protein targets a single base within a DNA duplex, a mechanism must exist to make the base accessible to the enzyme. For prokaryotic methyltransferases (and other DNA-modifying enzymes), crystal structures have shown that the target cytosine swings out of the helix completely and rotates through 180° on binding to the enzyme (Figure 4). It is thought that this base flipping mechanism is also employed by mammalian methyltransferases.
Mutagenesis and DNA repair of 5-methylcytosine
When cytosine is mutated to uracil by spontaneous deamination, the DNA glycosylase enzyme UDG (uracil DNA glycosylase) reverses the damage, in a base excision repair mechanism. When the equivalent deamination reaction occurs on 5-methylcytosine, however, the product, thymine, is not repaired by DNA repair enzymes (and 5-methylcytosine is an order of magnitude less susceptible to deamination than cytosine) (Figure 5). This has led to CpG dinucleotides becoming very much rarer in the genome than would be expected statistically (except at CpG islands, where no methylation, and therefore no mutation to thymine, occurs).
DNA hydroxymethylation
If 5-methylcytosine (mC) is the 'fifth base' in the genone 5-hydroxymethylcytosine (hmC) is the 'sixth base'. The oxidation of 5-methylcytosine (mC) to 5-hydroxymethylcytosine (hmC) is carried out by TET enzymes, members of the 2-oxoglutarate oxygenase family. It it proposed that this hydroxylation of 5-methylcytosine might the first step in an active pathway for DNA demethylation.
DNA demethylation
Methylation of cytosine bases was initially thought to be irreversible, and no direct DNA demethylase enzyme has been identified; but DNA demethylation is now known to be an important process.
DNA demethylation is necessary for the reactivation of silenced genes, and in 'cleaning the genomic slate' during embryonic development (this allows embryonic stem cells to differentiate into any cell).
Passive DNA demethylation
It is easy to envisage how DNA demethylation might occur across generations, through a loss of methylation information during DNA replication. An absence of maintenance methylation following DNA replication will result in globally demethylated DNA.
The maintenance DNA methyltransferas DNMT1 does not recognize 5-hydroxymethylcytosine, so a possible pathway for more specific passive methylation starts with the oxidation of 5-methylcytosine to 5-hydroxymethylcytosine. 5-Hydroxymethylcytosine has the same base-pairing chemistry as cytosine and 5-methylcytosine, so it will be replicated to cytosine; but no subsequent methylation/hydroxymethylation will occur. The overall result is demethylation.
Both of these mechanisms of passive cytosine demethylation require DNA replication. Such replication-dependent pathways are of no use if demethylation is required before the next round of DNA replication. Despite the apparent lack of a mammalian DNA demethylase enzyme, a pathway involving active (replication-independent) demethylaton has recently been proposed.
Active DNA demethylation
In plants, active DNA demethylation is achieved through base excision repair, beginning with the hydrolysis of the N-glycosidic bond of 5-methylcytosine by a specific DNA glycosylase. However, no demethylation pathway involving a 5-methylcytosine-specific DNA glycosylase has been identified in mammals. If 5-methylcytosine is first converted into another base, a base excision repair pathway might be feasible.
Following the oxidation of mC to hmC, further oxidation to 5-formylcytosine (fC) and then 5-carboxycytosine (caC) has been observed, also catalyzed by TET enzymes. These 'seventh' and 'eighth' bases have been detected in cells, and may be important epigenetic states in their own right. Two alternative mechanisms have been suggested for conversion of 5-carboxycytosine to cytosine: direct decarboxylation of 5-carboxycytosine, catalyzed by an as yet unidentified decarboxylase; or base excision repair, initiated by excision of 5-carboxycytosine by a thymine DNA glycosylase (TDG). Another pathway has been suggested, in which enzyme-catalyzed deamination of cytosine is followed by mismatch repair.
Histone modification
Although histones do not interact with polymerase enzymes directly, their modification can affect the way DNA wraps around them and thereby influence which genes are expressed. Histone modifications are necessary for recruiting cofactors and for polymerase binding, and for maintaining chromatin stability. Most modifications of histones occur at their unstructured, alkaline N-terminal tails. Important histone modifications include acetylation (at lysine residues) and methylation (at lysine and arginine residues) (Figure 7).
Histone acetylation
Histone acetylation is the most widely studied epigenetic protein modification. Acetylation of specific lysine residues in histone tails is associated with gene activation. Lysine acetylation, catalyzed by histone acetyltransferases (HATs), neutralizes the positive charge on the lysine residues. This charge neutralization is thought to reduce affinity between histones and DNA, opening up access to DNA for transcription factors and polymerases, and therefore enhancing transcription.
In histone acetylation, a conversed glutamate residue acts as a general base, activating the lysine ε-amino group for nucleophilic attack on the carbonyl group of acetyl CoA. A tetrahedral intermediate forms, and then collapses with the loss of coenzyme A (CoASH), to general acetyl lysine (Figure 8).
Histone deacetylation
Lysine acetylation is reversible: deacetylation, catalyzed by histone deacetylases (HDs or HDACs) represses transcription. The deacetylation of acetyl lysine involves nucleophilic attack of water on the acetyl carbonyl. The mechanism of activation of water is different for different deacetylases enzymes. There are four classes of histone deacetylases (I-IV), that catalyze the deacetylation of acetyl lysine via different mechanisms: classes I, II and IV use an active-site metal-dependent mechanism (Figure 9), while class III HDACs operate using a nicotinamide adenine dinucleotide (NAD+) dependent catalytic mechanism.
Histone methylation
The regulatory role of lysine methylation is complex: methylation of some lysine residues is associated with transcription, while methylation of other lysines is associated with repression of transcription.
Histone lysine residues undergo methylation at the N(ε) atom, catalyzed by lysine methyltransferases (KMTs). Histone Lysine methyltransferases catalyze mono-, di- and trimethylation of lysine (Figure 10).
The source of the methyl group is S-adenosyl-l-methionine (AdoMet), which is converted to S-adenosyl-l-homocysteine (AdoHcy) in the reaction (Figure 11).
Histones can also be methylated at arginine residues. As with lysine methylation, the regulatory role of arginine methylation is complex.
Histone demethylation
As with acetylation, lysine methylation is reversible; and lysine demethylation, catalyzed by lysine demethylases (KDMs), is an important epigenetic process.
The lysine demethylases fall into two broad classe: the LSD1/KDM (lysine specific demethylase) family and the JHDM (jumonji histone demethylase) family. The mechanisms of both classes of enzymes require an oxidative step. The mechanism of lysine demethylation catalyzed by enzymes of the LSD1/KDM1 family is illustrated in Figure 12.
Epigenetics in cancer
The discovery of the role epigenetics plays in cancer has made epigenetics an area of huge recent interest, and understanding it has led to new cancer treatments.
Epigenetic modifications are essential in the development and function of healthy cells. Changes in the epigenome (for example, in the methylation pattern of DNA), leading to incorrect activation or inactivation of sigalling pathways, are a hallmark of cancer. Cancer was long seen as a genetic disease, but it has become clear recently that epigenetic factors are equally important.
Changes to the epigenome in tumour cells
DNA methylation
In normal cells, DNA methylation occurs at CpG sites, but CpG islands remain unmethylated. In tumour cells, the level of DNA methylation at CpG sites is generally low (hypomethylation), while CpG islands may be methylated (hypermethylation). DNA hypomethylation leads to aberrant activation of genes.
Histone modification
Specific histone modifications are associated with tumour formation, including deacetylation of histone 4 lysine 16 (H4K16), mediated by histone deacetylases (HDACs). It is thought that this histone deacetylation results in the repression of tumour-suppressor genes. Cancer cells also display changes in the methylation patterns of lysine residues including H3K9 and H3K27.
Cancer treatment: epigenetic therapy
While genetic mutations are irreversible, epigenetic modifications are, to varying degrees, reversible. This opens up the possibility of reversing epigenetic modifications in cancer cells to restore the cells to their healthy state. The goal of epigenetic therapy in cancer treatment is to restore a distorted epigenome to a 'normal' epigenome.
DNA methylation inhibitors
The cytotoxic nucleoside analogues 5-azacytidine (azacitidine) and 5-aza-2'-deoxycytidine (decitabine) have recently been approved for the treatment of some types of cancer. Azacytidine and decitabine are incorporated into the DNA of rapidly growing tumour cells during DNA replication and, after replication, inhibit methyltransferases. This loss of methylation leads to activation of tumour-suppressor genes, inhibiting tumour growth.
Non-nucleoside compounds that can inhibit DNA methyltransferases without being incorporated into DNA are being pursued, but no potent inhibitors have yet been found.
Histone deacetylase inhibitors
The restoration of histone acetylation patterns has been shown to correlate with antitumour activity, and histone deacetylase (HDAC) inhibitors have been investigated to this end. Suberoylanilide hydroxamic acid (SAHA; Vorinostat; Zolinza), an inhibitor of some classes of HDACs, was approved for the treatment of cutaneous T cell lymphoma in 2006.